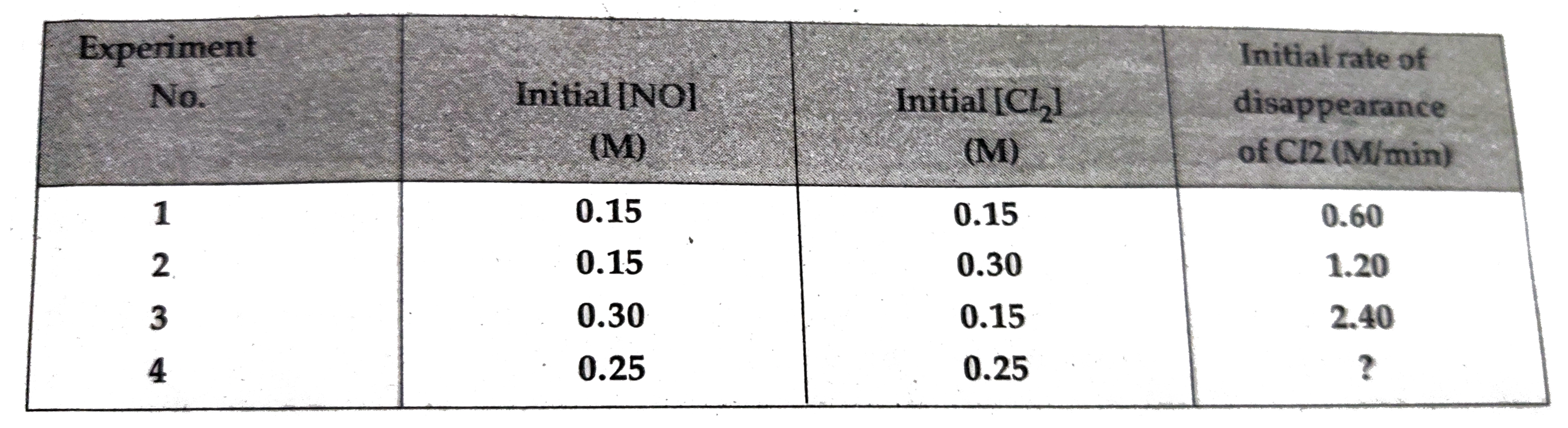
Calculate the enthalpy change for the process `C Cl_(4)(g) rarr C(g)+4Cl(g)` and calculate bond ... - YouTube

Calculate the enthalpy change for the process CCl4(g) → C(g) + 4Cl(g) and calculate bond.... - YouTube

The gas-phase reaction Cl(g)+HBr(g)--->HCl(g)+Br(g) has an overall enthalpy change of -66kJ. The activation energy for the reaction is 7kJ. a) Sketch the energy profile for the reaction and label E | Homework.Study.com

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (

The first ionisation energy of Li is 5.4 eV and electron affinity of Cl is 3.61 eV. What is the value of Δ H(in kJ/mol) for the following reaction? Li( g) + Cl(g)→

Mn²⁺ ion coordination mechanism in 4-Cl-BQ electrodes a The first GCD... | Download Scientific Diagram
16. Calculate the enthalpy change for the process CCl4(g)————C(g)+4Cl(g) And calculate bond enthalpy of C Cl in CCl4(g). Δ vapH(CCl4)=30.5 kj /mol Δ fH(CCl4)= 135.5 kj/mol Δ aH(C)=715.0 kj/mol Δ aH(Cl2)=242 kj/mol

Le grand-père, ou, Les deux âges . ^^^^ g^— ^?=^-:=4 l^^^EE^ J^ m ^ p ^^^ fe^^^ Ts ic me siUMi W- ^m m^^m ^^s 1 (• cl IC ^^ ^^